Feldspar is a common mineral that makes up about half of the Earth’s crust. It has some unexpected properties beyond just being part of the soil. In the Earth’s atmosphere, feldspars have a surprisingly important job: they help make clouds.
The tiny dust from this mineral, when it’s carried through the air, affects how clouds form. Water molecules stick better to feldspar dust than to other particles. So, the small bits of this mineral floating in the atmosphere turn into great ‘seeds’ for water molecules to attach to and freeze, eventually forming a cloud.
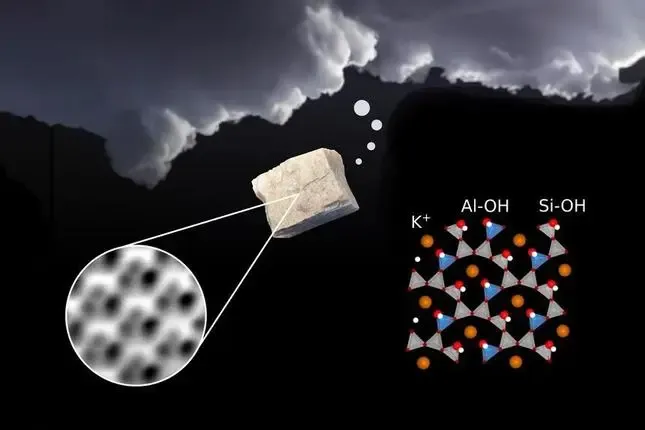
Scientists aren’t sure why feldspar is so good at binding water and helping clouds form, and this is a topic of recent research.
Researchers at the Technical University of Vienna (TU Wien) have used a highly sensitive atomic microscope to demonstrate that the unique geometry of the feldspar surface provides the perfect anchoring point for the OH groups of hydrogen and oxygen and subsequently for water.
Microcline mineral reacts with water
Scientists have conducted a detailed study on microcline feldspar (001) and its interaction with water, shedding light on atomic-scale details. In simpler terms, microcline is a type of mineral, and the study looked at how it behaves when exposed to water.
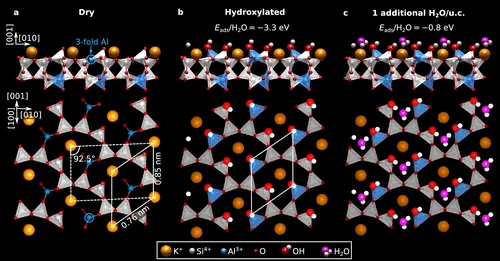
The researchers found that when the dry surface of microcline is cleaved, it essentially breaks some bonds on the surface, leaving oxygen atoms on the top of silicon atoms and creating undercoordinated aluminum. Water readily reacts with this surface, dissociating and forming hydroxyl groups. Even without intentional water supply, the surface of microcline reacts with water at room temperature, becoming fully hydroxylated.
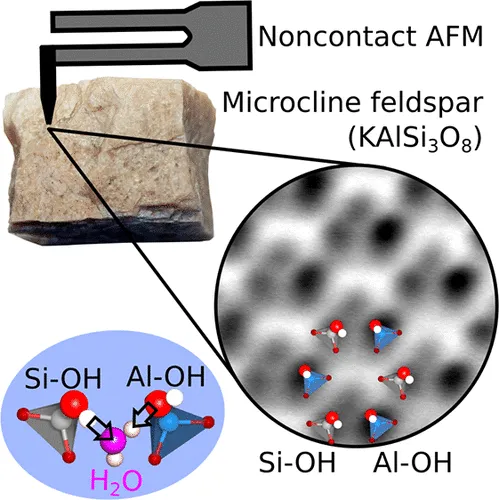
The study used advanced techniques like Atomic Force Microscopy (AFM) and X-ray Photoelectron Spectroscopy (XPS) to analyze the surface at the atomic level. The AFM images revealed a honeycomb pattern on the hydroxylated surface, and simulations matched well with experimental data.
Furthermore, the study explored how additional water molecules adsorb on the fully hydroxylated surface. The results indicated that the molecules remain undissociated and form hydrogen bonds with the surface.
The findings have implications for understanding how water interacts with mineral surfaces, providing insights into the atomic-scale processes. The study suggests that microcline, a specific type of feldspar, readily reacts with water, and the ordered arrangement of atoms on its surface influences the adsorption of water molecules.
Images with atomic resolution
“Professor Ulrike Diebold from the Institute of Applied Physics at the Technical University of Vienna, who led the project, says, ‘The researchers were considering several ideas about why feldspar is such an effective nucleation seed. The potassium atoms contained in the feldspar could be a reason, or perhaps certain defects in its crystalline structure.”
The TU researchers found out by using a very sensitive atomic microscope, which scans the surface of the crystal point by point. The force between the tip and the surface produces a high-resolution image, in which they can precisely determine the position of each atom.
“Giada Franceschi, the first author of the study, says, “We placed a piece of feldspar in the vacuum chamber of the microscope and divided it in half to obtain a flawless and clean surface. The images of the surface perplexed us as they looked different from what common theories had predicted.”
An optimal connection: the hydroxyl layer
Giada Franceschi quickly discovered the cause: small water encrustations in the rock were the culprits. When the stone breaks, it releases some water vapor. The vapor sticks to the newly split surface, and the water molecules break apart to form hydroxyl (OH) groups. “Under the microscope, you don’t examine the feldspar’s surface itself, but rather a surface blanketed with hydroxyl groups,” Franceschi explains. “In nature, a hydroxyl layer also covers the surface of feldspar.”
The geometry of the feldspar crystal positions these hydroxyl groups ideally as anchoring points for water molecules. Water molecules can attach to the hydroxyl groups like precisely fitting building blocks. Thus, the hydroxyl layer establishes the perfect connection between the feldspar and the adhering water in the form of ice.
Ulrike Diebold says, “You can establish the connection very easily and quickly, and it remains very stable. If you want to remove the hydroxyl layer from feldspar, you have to heat it to high temperatures. The findings also receive support from computer simulations.”
“The results allow us to gain insights into why our atmosphere’s specific crystals make suitable cloud-forming nucleation seeds. Understanding the physics of cloud formation becomes particularly crucial in the face of climate change. The research project at the Technical University of Vienna sometimes necessitates that we delve deeper into the world of atoms.”
Support us to keep independent environmental journalism alive in India.
Keep Reading
What is Green Hydrogen? Could it change energy in South Asia?
Blue hydrogen is worst for climate: study
How Increasing space traffic threatens ozone layer?
Hydro Fuel Market: India’s current scenario and the future ahead
Natural Gas is a Misleading term, It is not Natural and clean at all
Follow Ground Report on X, Instagram and Facebook for environmental and underreported stories from the margins. Give us feedback on our email id greport2018@gmail.com.
Don’t forget to Subscribe to our weekly newsletter, Join our community on WhatsApp, and Follow our YouTube Channel for video stories.









