The U.S. Food and Drug Administration (FDA) has approved Traumagel, a plant-based gel that promises to stop moderate to severe bleeding rapidly. This gel advances trauma care, particularly for life-threatening injuries like gunshot and stab wounds. Traumagel will impact emergency medical services, the military, and remote care by preventing death from blood loss.
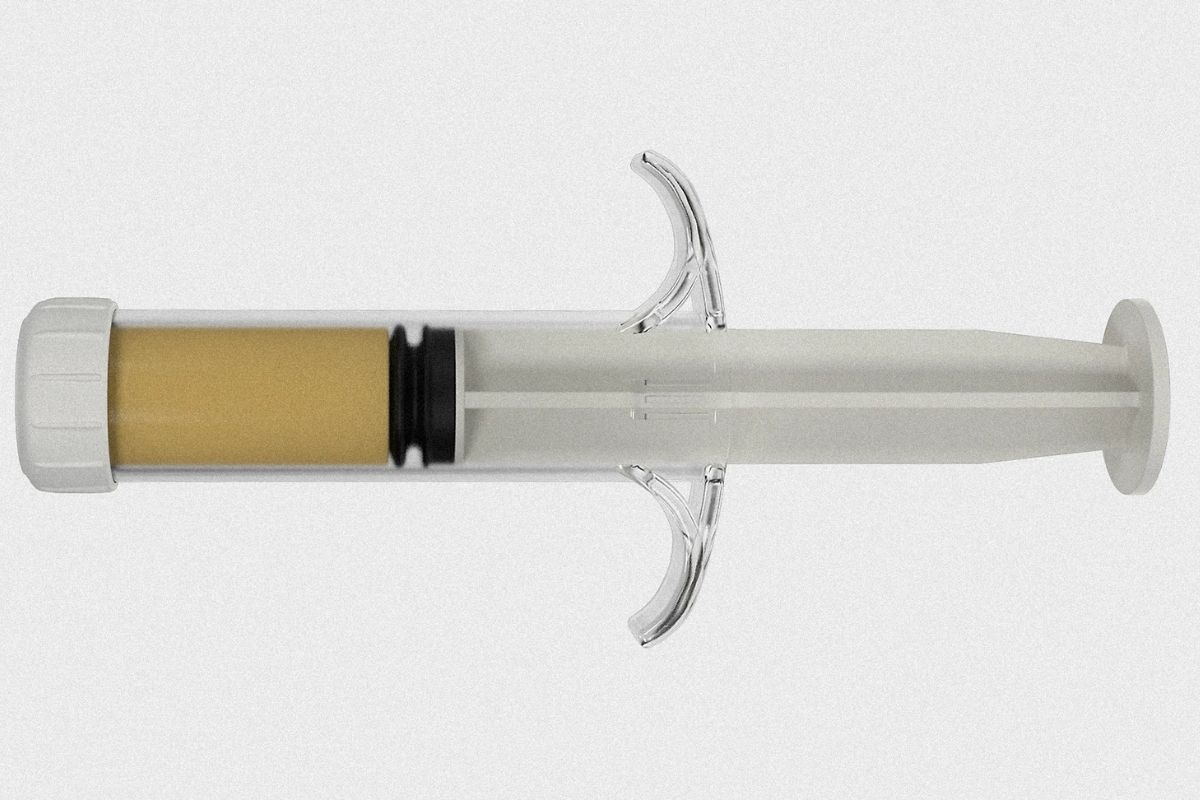
Developed by Cresilon, a Brooklyn-based biotechnology company, Traumagel has received FDA clearance for temporary external use, making it the first gel-based treatment for controlling severe bleeding. The product is expected to be available by late 2024 and will be distributed in pre-filled syringes for easy and fast application during critical trauma events.
The FDA has approved Traumagel.
a plant-based gel that quickly stops bleeding and helps the body’s natural clotting process.
This could be vital for first responders, military personnel, and remote care.
Thoughts?#FDAApproval #TraumaCare pic.twitter.com/apYuQADkSR
— Zain Khalpey, MD, PhD, FACS (@ZainKhalpey) October 2, 2024
With Traumagel, first responders and healthcare professionals can rapidly control bleeding, a vital feature in saving lives during emergencies. According to Cresilon, the gel helps the body’s natural clotting process, reducing the need for traditional methods like packing wounds with gauze.
What is FDA-approved plant-based Traumagel?
Traumagel is the first plant-based hemostatic gel designed to stop bleeding quickly. Cresilon, the company behind it, developed it for military, government health agencies, and emergency responders dealing with life-threatening bleeding. In critical situations, Traumagel can be the difference between life and death.
The gel uses proprietary hydrogel technology that is effective and user-friendly. The FDA approved it for temporary external use in moderate to severe bleeding. Traumagel is expected to improve bleeding wound management in emergencies.
How does Traumagel work?
Traumagel forms an instant clot when applied to a bleeding wound. Delivered through a syringe directly into the injury, it helps the patient’s body clot blood faster than traditional methods like gauze packing. The gel fills the wound’s crevices, ensuring comprehensive coverage and faster clotting.
Another advantage is its ease of use. The gel doesn’t require pressure or direct contact with the blood, making it safer for healthcare providers. This feature minimizes the risk of injury from fragments in wounds, like bones or bullets. The gel’s effectiveness and rapid action make it suitable for high-stakes trauma situations like gunshot or stab wounds.
The FDA classifies medical devices by risk. Traumagel, like other moderate-risk devices, received FDA clearance instead of approval. FDA clearance, granted under the 510(k) process, means the product is as safe and effective as an already-marketed device. This applies to Class I and Class II devices.
FDA approval is reserved for Class III devices, considered higher risk. Approval signifies the benefits outweigh known risks after the agency’s rigorous evaluation. Both clearance and approval demonstrate the product’s safety, but they apply to devices with different risk levels.
Support us to keep independent environmental journalism alive in India.
Keep Reading
Part 1: Cloudburst in Ganderbal’s Padabal village & unfulfilled promises
India braces for intense 2024 monsoon amid recent deadly weather trends
Follow Ground Report on X, Instagram and Facebook for environmental and underreported stories from the margins. Give us feedback on our email id greport2018@gmail.com.
Don’t forget to Subscribe to our weekly newsletter, Join our community on WhatsApp, and Follow our YouTube Channel for video stories.